Specially-prepared revision booklets have been produced by Bangor University with the support of a National Science Academy grant from the Welsh Government. The booklets contain revision material prepared by experienced Science teachers for the University’s GCSE revision courses, and have been highly praised by both schools and pupils.
Click here to access the Bangor University website where you can download revision guides as pdf files for all three Science area covering the topics for Unit 1 (Year 10) and Unit 2 (Year 11).
This is the blog of the Cowbridge Comprehensive School Chemistry department. It's purpose is to provide material which will be of direct help in studying Chemistry and will also provide interesting links to all things chemical. Any images/video clips used are strictly for educational (non-profit making) purposes, if you feel there are any copyright infringements or do not wish for the images to be used, please contact us and we will remove them.
Thursday, 5 June 2014
Monday, 7 April 2014
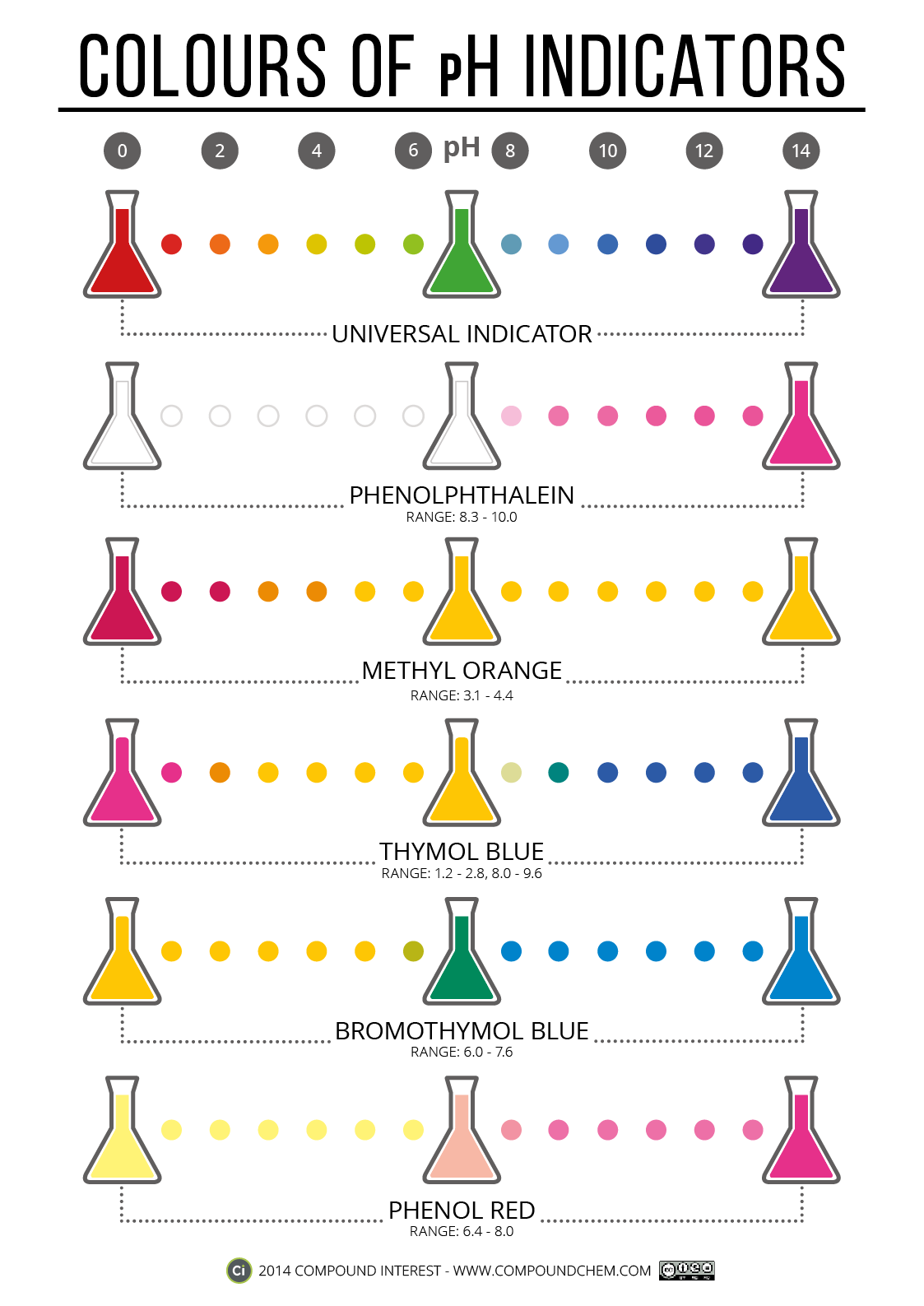
Indicators - Colours and pH ranges
Compound Interest have produced a good poster on Indicators, the colours they are in acid and alkaline solutions, along with the chemistry behind them. click on the picture below to link to Compound Interest's blog.
Wednesday, 2 April 2014
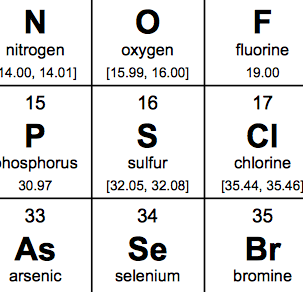
How do you spell element 16? Sulphur or Sulfur?
Having been part of the education system as a pupil and then a teacher, I have been confronted by the question how do you spell element 16? Sulphur or sulfur?
Even as I write this, the spell check on this blog is underlining the word sulfur to tell me it is wrong! When auto correct is working it automatically corrects sulfur to sulphur. I have always spelt it sulphur having been told to do so by teachers and lecturers alike over many years, and have cheerfully promoted that spelling with pupils.
However an article on 'the chronicle flask' blog has finally made me change my mind. It identifies that IUPAC (The International Union of Pure and Applied Chemistry) have deemed the spelling of element to be sulfur, and as they set the rules that is the way it should be.
Click on the picture to read the full article.
Even as I write this, the spell check on this blog is underlining the word sulfur to tell me it is wrong! When auto correct is working it automatically corrects sulfur to sulphur. I have always spelt it sulphur having been told to do so by teachers and lecturers alike over many years, and have cheerfully promoted that spelling with pupils.
However an article on 'the chronicle flask' blog has finally made me change my mind. It identifies that IUPAC (The International Union of Pure and Applied Chemistry) have deemed the spelling of element to be sulfur, and as they set the rules that is the way it should be.
So element 16 is sulfur!
Click on the picture to read the full article.
Wednesday, 26 March 2014
Spectroscopy in a suitcase
On Tuesday 25th of March Cardiff University visited the school on behalf of the Royal Society of Chemistry to deliver a workshop on spectroscopy to the year 12 Chemistry pupils. Spectroscopy forms an important part of the F322 AS module. Dr Dayna Mason introduced the topic and got the pupils involved in interactive tasks to identify unknown organic compounds by using an infra-red spectrometer (a piece of equipment that they would only be able to use at university level) to test liquid samples.
The pupils compared their experimental findings with mass spectrogram results and databases to find their unknown organic compounds.
 |
| Pupils testing samples on the IR-Spectrometer |
For more information on spectroscopy and 'spectroscopy in a suitcase' visit the RSC website - http://www.rsc.org/learn-chemistry/collections/spectroscopy
Tuesday, 25 March 2014
Fluoridation of water
Fluoride is known to help prevent tooth decay. Sodium fluoride can be added to toothpaste and water in order to do this. GCSE pupils need to be aware that there are arguments for and against this process. Click on the picture below to link to a BBC news article where this is discussed. Also have a look at the related stories as it will provide background information and supplement your understanding from lessons.
Monday, 24 March 2014
Maths in Chemistry!
If you need a little more help covering the mathematical areas of Chemistry then click on the picture below. It links to a website created by the Royal Society of Chemistry and Pfizer which has useful hints and practice questions to cope with common mathematical themes.
Click on the compass to find an area to work on, or use the video demos to help.
Click on the compass to find an area to work on, or use the video demos to help.
Chemistry in everyday use
Whilst you might be aware of the main elements in the periodic table - group 1, group 2, the transition metals, the Noble gases etc. there are some very useful metals found at the very bottom of the table belonging to a group known as the lanthanides. Click on the picture below to read about some of their unique and interesting uses.
Sunday, 23 March 2014
Crude Oil Revision
Some useful revision videos on crude oil and its uses from Fuse School.
This video shows the forces of attraction between chains of hydrocarbons, the longer the chain the greater the force. But it doesn't just happen end to end as the video suggests, rather across the whole molecule.
|
|
|
Short
chains have fewer carbon atoms so there are attractions between the chains as
a result less energy is needed to separate the molecules. Low boiling point.
|
Long
chains have more carbon atoms so there are more attractions between the
chains as a result a greater amount of energy is needed to separate the
molecules. High boiling point.
|
Nanoscience - Buckyballs, Fullerenes and Nanotubes,
Fuse School have produced a good video explaining some different types of nanoparticle. This is particularly useful for Year 11 studying WJEC Unit C2.1. Click on the picture below to access the video.
Monday, 17 March 2014
Electrolysis of lead bromide
As seen in class molten lead bromide can be separated into lead and bromine using electrolysis. It must be molten so that the ions are free to move and conduct electricity. When an electric current is applied the following half cell reactions take place:
At the anode (+ve electrode) the negative bromide ions are attracted, where they lose electrons and become bromine gas:
At the cathode (-ve electrode) the positive lead ions are attracted, they gain electrons and become molten lead.
At the anode (+ve electrode) the negative bromide ions are attracted, where they lose electrons and become bromine gas:
2Br-(l) → Br2(g) + 2e-
This is an oxidation reaction.At the cathode (-ve electrode) the positive lead ions are attracted, they gain electrons and become molten lead.
Pb2+(l)
+ 2e- →
Pb(l)
This is a reduction reaction.
Remember OIL RIG and you can't go wrong with electrolysis. Oxidation Is Loss of electrons, Reduction Is Gain of electrons.
Check out this YouTube video below of the electrolysis of lead bromide:
Remember OIL RIG and you can't go wrong with electrolysis. Oxidation Is Loss of electrons, Reduction Is Gain of electrons.
Check out this YouTube video below of the electrolysis of lead bromide:
Name the new International Space Station mission
The European Space agency is asking people to choose a name for British astronaut Tim Peake's next mission. It would be great if we had a winner from Cowbridge! Click here to have a go.
The closing date is the 4th April so get thinking!
The closing date is the 4th April so get thinking!
Thursday, 13 March 2014
WJEC AS Unit 2 - Functional Groups
Year 12 (and 13) if you want reminding what the organic functional groups look like, whether it appears in the molecule name as a prefix or a suffix click on the picture below and download the pdf file.
Haloalkanes and the same as halogenoalkanes. You don't need to know the nitrile, acyl chloride or ether functional groups.
Haloalkanes and the same as halogenoalkanes. You don't need to know the nitrile, acyl chloride or ether functional groups.
Wednesday, 12 March 2014
Organic Reaction Maps
WJEC A2 Unit 4 - Organic Reaction Maps
Year 13 pupils, check out the compound interest blog. They have produced a couple of excellent organic reactions pathway posters (for both aliphatic and aromatic) which will be useful for revising the synthesis topic for F324. Some of the reactions are not relevant to the OCR Chemistry A course so you will have to cross reference them with the syllabus. Click on the pictures below to get to the blog itself.
Sunday, 9 March 2014
The Thermit Reaction
Displacement Reactions - The Thermit Reaction
10X Triple Chemistry have been looking at displacement reactions. These reply upon a more reactive chemical displacing (kicking out) a less reactive chemical from a compound. The pupils have carried these types of reaction out on a test tube level. A more exciting example though is the Thermit reaction:
Iron
oxide
|
+
|
Aluminium
|
-->
|
Aluminium
oxide
|
+
|
Iron
|
Fe2O3(s)
|
+
|
2Al(s)
|
-->
|
Al2O3(s)
|
+
|
2Fe(l)
|
Aluminium is above iron in the reactivity series so it displaces the iron in such a highly exothermic reaction that the iron produced is molten. The video below shows how the reaction worked in class. You can see the molten iron falling in the slow motion reply.
Friday, 7 March 2014
Thursday, 6 March 2014
More Nanoscience!
Another good article on nanoscience form the guardian
Click on the picture below to link to the article:
Have you say on nanoscience at:
Click on the picture below to link to the article:
Have you say on nanoscience at:
Monday, 3 March 2014
The Elements of the Periodic Table
Periodic Table Challenge
Mr Pullen set a challenge for his 7-6 class to see who could sing "The Elements" song originally written by Tom Lehrer. This challenge was duly accepted by Ed Sunderland who performed this recently for his class:
So impressed by this Mr Pullen awarded him with an the photographic Elements card sort prize:
Well done Ed!
Just for comparison here is the original:
Saturday, 1 March 2014
Nanoscience in every day use

Click here for the guardian article
Thursday, 20 February 2014
Year 12 - Spectroscopy in a Suitcase - Tuesday 25th March 2014
On Tuesday 25th March Cardiff University are coming into school to carry out a Royal Society of Chemistry interactive workshop on Infra-red spectroscopy. This will help complement an essential topic of F322 - Module 2: Alcohols,
Halogenoalkanes and Analysis. The workshop will be split into two sessions and held in A3B during lessons 4 and 5, it is open to all AS Chemistry pupils. See Mr Cox for further details.
Check out the introductory video below:
Check out the introductory video below:
Welcome to the new Chemistry department blog!
It is designed for pupils, parents and teachers alike to gain an insight into activities in the Chemistry department. This will be a tool for information about courses and topics. We will be posting revision materials and links which GCSE and A level pupils will find interesting and hopefully helpful. We will also post interesting facts, videos and news articles on Chemistry and other Science related topics.
We would like this to be a resource which you will find both informative and useful. There will be posts that strike up debate and we would encourage you to make comments and discuss the issues that have been raised. However commenting on members of staff, or being derogatory towards other users will not be tolerated.
It is designed for pupils, parents and teachers alike to gain an insight into activities in the Chemistry department. This will be a tool for information about courses and topics. We will be posting revision materials and links which GCSE and A level pupils will find interesting and hopefully helpful. We will also post interesting facts, videos and news articles on Chemistry and other Science related topics.
We would like this to be a resource which you will find both informative and useful. There will be posts that strike up debate and we would encourage you to make comments and discuss the issues that have been raised. However commenting on members of staff, or being derogatory towards other users will not be tolerated.
Subscribe to:
Comments (Atom)










.gif)


